The Genesis: A Toxicity Crisis
In October 2024, a university engineering department in Colombia reached out to us via a mutual connection regarding a critical water quality issue.
This wasn't just about taste or odor; it was a toxicology issue. A remote community named El Juncal was relying on a water source containing 2.4 mg/L of fluoride.
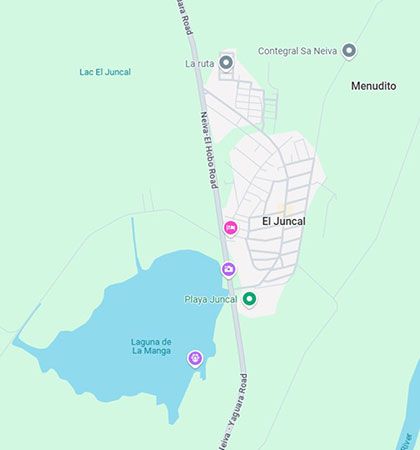
To put that number in perspective, it is more than double the maximum acceptable value of 1.0 mg/L established by the Colombian National Standard (Resolución 2115 de 2007).
The community was already exhibiting signs of long-term exposure. According to the definitive National Research Council (NRC) review on fluoride toxicity, sustained consumption at these levels is directly linked to severe dental degradation and skeletal fragility.
To arrest the health crisis, the university set an aggressive remediation target of <0.5 mg/L, going well beyond regulatory compliance to ensure consumer safety.
Inquiries like this are not unusual. Most come from engineers around the world who need help designing a custom solution for water or wastewater challenges that off-the-shelf equipment can't handle. That is exactly what we are known for. We see unique cases every week, but this one brought a new variable: fluoride removal was a personal first for me.
Through our interpreter and mutual friend, I accepted the request and sent back a request for the essentials: source water details, flow rate, a full constituent profile, and the status of any current infrastructure.
A Feasibility Check: The ROM
While waiting for the hard data, I mocked up a preliminary system design to generate a rough order of magnitude (ROM) estimate. This is a standard "Go/No-Go" test: before we invest heavily in engineering, we need to verify if the prospect has the budget for a premium, American-built system.
Working with limited details and assuming we were pulling from a nearby lagoon visible on satellite maps, I designed for a worst-case "surface water" scenario. I threw the kitchen sink at it:
- Headworks: Two skidded pumps with variable frequency drives (VFD), flocculation tubes, and chemical dosing pumps with a polymer makedown unit. Assumed they had a tank of some sort to repurpose.
- Clarification: A dissolved air flotation (DAF) unit to handle heavy turbidity and solids.
- Polishing: Pressure vessels, activated glass filtration media (AGFM), bag filters, ceramic nanofiltration, and a disinfection station. Assumed they had another tank for treated water nearby.
- Support: Control panels, sludge transfer pump, air compressor, and a 5,000-gallon fiber reinforced plastic (FRP) sludge holding tank.
The Bottom Line
When we factored in engineering, freight, travel, installation, local taxes, insurance, and security escorts, the ROM landed at $1,865,000.
- Schedule: I proposed a standard timeline of 4 weeks for drawings and submittals followed by 16 weeks for fabrication.
The Green Light
The prospect was unfazed by the time and cost. On January 24, 2025, they provided some of the requested data. This revealed the first major pivot of the project: we weren't treating dirty lagoon water.
Gaining Clarity
Although we did not gain the comprehensive water analysis or much of anything I had requested, we did receive a brief email that corrected my assumption regarding the water source and provided some essential design parameters.
We were treating well water rather than surface water from the lagoon. This distinction meant the heavy clarification equipment was no longer necessary. However, the email introduced a new variable in the data. Molybdenum.
- Flow Rate: 554 GPM
- Fluoride: 2.40 mg/L (Target: <0.5 mg/L)
- Molybdenum: 0.09 mg/L (Target: 0.07 mg/L)
This was the first time molybdenum entered in the conversation. The required reduction was small but needed to be addressed. We were looking at a fairly high flow application in a remote location where logistical support would be limited.

Service Capacity Analysis
| Parameter | Value | Unit | Logic / Formula |
|---|---|---|---|
| System Flow Rate | 554 | GPM | Design capacity |
| Operational Availability | 1,440 | Min/Day | Continuous 24-hour operation (feeding storage) |
| Total Daily Production | 797,760 | Gallons/Day | 554 GPM x 1,440 min/day |
| Rural Consumption Estimate | 50 | GPD/Person | Conservative estimate for rural communities |
| Total Population Served | ~15,955 | People |
The Ion Exchange (IX) Strategy
My first instinct was to explore IX. After browsing the Purolite catalog, I reached out to a friend there to ask about their A200 resin. We zeroed in on this one because it is a polystyrenic gel Type II strong base anion in the chloride form.
We specified the resin in the chloride form (Cl-) to ensure that the ion exchange reaction releases neutral chloride ions rather than hydroxide ions (OH-) thereby preventing a drastic upward pH excursion in the effluent. This configuration simplifies the chemical architecture by eliminating the need for post-treatment acid neutralization and allows for regeneration using standard sodium chloride brine instead of hazardous caustic soda.
We chose Type II specifically because it offers higher operating capacity and superior regeneration efficiency compared to Type I variants. When dealing with monovalent anions like fluoride, you need that high-capacity exchange to drive the concentration down hard. A properly applied strong base anion resin is capable of a 90% to 95% reduction in fluoride.
However, while the A200 is a workhorse, standard strong base anion resins suffer from a hierarchy of affinity. They naturally grab sulfates and nitrates long before they look at fluoride. We didn't want a resin that would load up on background anions and let the fluoride slip through, so I pivoted to Itochu to evaluate a dedicated fluoride-selective resin: the INDION RS-F.
My preliminary design involved treating with granular activated carbon (GAC) first to protect the resin from oxidation and fouling, followed by the ion exchange stage.
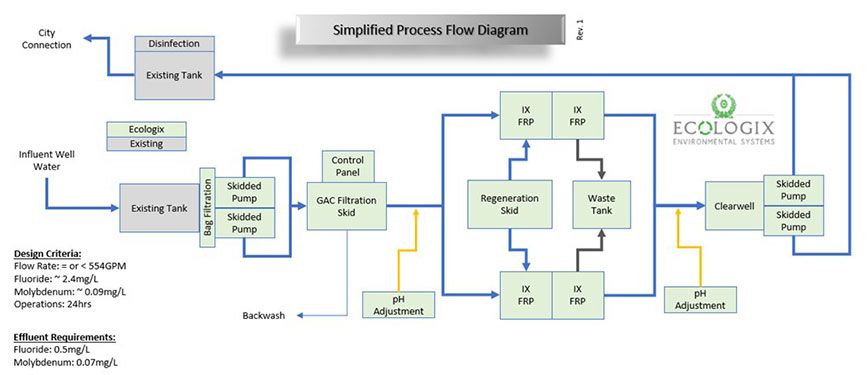
While exploring the IX options, I updated the process flow and equipment list to reflect the new strategy. This shifted the focus from heavy clarification to a dedicated adsorption and exchange train.
- Pre-treatment 1: Raw water holding tank and skidded pumps.
- Pre-treatment 2: GAC filtration to protect the resin from oxidation and fouling.
- Primary treatment: Resin filtration vessels (strong base anion).
- Post-treatment: Clearwell, secondary skidded pumps, and a final holding tank with disinfection.
The change in technology and the addition of the polishing stage bumped the ROM estimate to $1,952,000.
The Reality Check
On paper, the INDION RS-F looked like the sniper we needed, however while specifying the new layout the solution quickly unraveled.
Keep in mind, we avoid complexity whenever possible because it kills deals and creates operational nightmares. If you sell a system that requires a dedicated chemical engineer to run, it fails. This specific site is in a remote location far from a major city, meaning experienced operators are unicorns.
To unlock the fluoride selectivity of the INDION RS-F at 126 m³/hr., the chemistry had to be perfect. This resin required a massive pH excursion: we would have to inject acid to drop the influent pH to ~5.0 to maximize adsorption, then inject caustic to raise it back to ~7.0 for distribution. That is two separate chemical feed systems introducing hazardous handling and potential failure points. Strike one and strike two.
Strike three came from the regeneration requirements. The loading calculations dictated a regeneration cycle every 27 hours using a significant volume of polyaluminum chloride (PAC). Between the aggressive pH manipulation and the logistical nightmare of hauling that much chemical to a remote site, the INDION RS-F concept was DOA.
The Reality of pH Adjustment
For the curious, executing those pH swings isn't just about dumping liquid into a tank. It requires a precise, high-maintenance control loop.
- The Drop (pH ~5): We would likely use sulfuric acid (H₂SO₄). While cost-effective, it introduces sulfate ions that increase the risk of scaling. Dosing requires strict alkalinity titration and jar testing to manage buffering capacity. We aren't just pouring it in; we are talking about corrosion-resistant peristaltic pumps, inline pH sensors, and tight proportional-integral-derivative (PID) control loops to prevent overshooting.
- The Rise (pH ~7): To neutralize the water post-treatment, we would inject sodium hydroxide (NaOH). This requires a mirrored setup: real-time monitoring, proportional chemical feed systems, and static mixers.
- The Risk: Beyond the chemistry, this turns the site into a hazardous zone. It necessitates secondary containment, spill kits, emergency alarms, and strict safety protocols. All of this for a remote resort with no dedicated staff.
The Pivot
With the resin option dead, we needed a new solution.
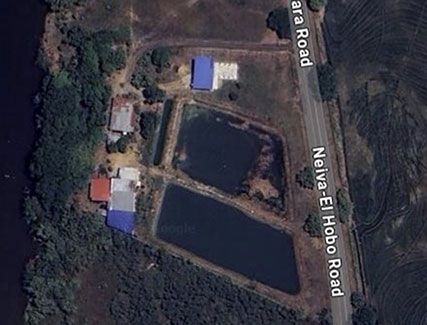
Things were getting serious, so we hopped on a call. The prospect finally handed over the specific GPS coordinates so I could see exactly what we were dealing with. Lo and behold, satellite imagery revealed an actual water treatment plant already sitting there. The things you learn after three months.
By February 10, 2025, I had a new design ready. After consulting with a colleague and digging through the alternatives, I moved away from complex chemistry and settled on a media I had never used before: bone char.
Why Bone Char is the Holy Grail
This is not just a different filter media; this changes the laws of engagement. Bone char is primarily composed of hydroxyapatite Ca₁₀(PO₄)₆(OH)₂, a biological calcium phosphate lattice.
The magic happens at the molecular level through a process called lattice substitution. The fluoride ion F⁻ is highly electronegative and chemically aggressive. When it encounters the hydroxyapatite matrix, it kicks out the hydroxyl group OH⁻ and takes its place in the crystal structure.
This converts the media into fluorapatite Ca₁₀(PO₄)₆F₂.
This isn't just surface adsorption; it is a chemical transformation. Fluorapatite is thermodynamically more stable and chemically insoluble than the original bone char. Once the fluoride locks into that lattice, it stays there.
Unlike the resin nightmare, this reaction runs efficiently at the water's natural, neutral pH. No sulfuric acid dosing. No caustic neutralization. No daily regeneration with PAC. We get high-selectivity removal in a passive, flow-through system that requires low operator intervention.

Bone Char - What Is It?
It looks like GAC. Black, gritty, and porous. But chemically, it is an entirely different animal. Standard activated carbon is almost 100% elemental carbon derived from coal, coconut shells, or wood. Bone char, however, is a composite material. It is essentially a carbon-coated skeletal structure.
| Component | Percentage | Function |
|---|---|---|
| Hydroxyapatite | 70% - 76% | The workhorse. This inorganic calcium phosphate lattice [Ca10 (PO4)6 (OH)2 ] provides the site for fluoride ion exchange. |
| Elemental Carbon | ~10% | The "skin." A thin layer of activated carbon covers the pore surfaces, providing adsorption for organics, taste, and odor. |
| Calcium Carbonate | 7% - 9% | Structural support and minor pH buffering. |
You don't just burn bones to get bone char; if you did, you would get white ash. The process requires pyrolysis which is a thermal decomposition in an oxygen-starved environment. The bones (usually cattle) are sun-dried and thoroughly cleaned to remove flesh and grease. The bones are sealed in iron retorts or kilns to prevent oxygen ingress. They are heated to ~ 700°C (1,292°F). The heat drives off the organic material (collagen and cartilage) as gas. Because there is no oxygen, the carbon doesn't burn away; instead, it deposits itself as a microscopic layer over the remaining honeycomb structure of the calcium phosphate.
Bone char does not "hold" fluoride like a sponge holding water (adsorption). It chemically incorporates it. The hydroxyapatite crystal has a hydroxyl group (OH-) that fits loosely in its structure. The fluoride ion (F-) is chemically similar in charge and radius but is more electronegative. When fluoride hits the media, it kicks out the hydroxyl ion and takes its place, effectively turning the bone char into natural fluorapatite (the same mineral found in tooth enamel).
Ca10 (PO4)6 (OH)2 + 2F- → Ca10 (PO4)6 F2 + 2OH-
Bone char isn't new technology. Around 1815 sugar refiners discovered that charred animal bones were incredibly effective at removing color from cane sugar syrup, turning it from brown to the stark white consumer sugar we see today. In 1948 the first major municipal attempt to remove fluoride from drinking water in the U.S. occurred in Britton, South Dakota. They used bone char. It was the "gold standard" for defluoridation until the 1970s, when cheaper synthetic aluminas and membrane technologies began to push it out of the municipal market.
Bone char is the definition of Waste-to-Value. It utilizes a massive waste stream from the meat industry (millions of tons of bones) that would otherwise fill landfills. It is a renewable resource, unlike coal-based carbons which are fossil-fuel derived.
The Final Design
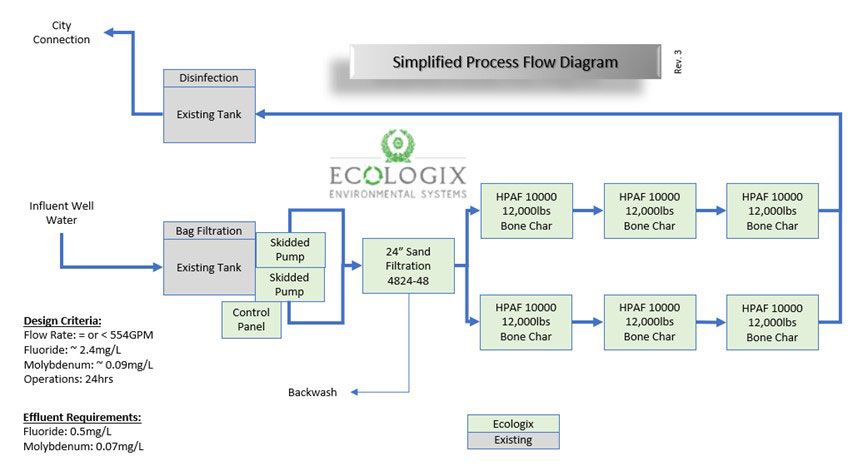
Armed with the new site information and the bone char solution we overhauled the layout.
- Integration: We utilized the client's existing bag filters on their influent tank.
- Pumping: We retained our VFD-equipped dual-skid pump configuration which gives us new pumps and flow control.
- Pre-treatment: The GAC units were unnecessary with the new approach, so we swapped them for a sand filtration skid to handle particulates.
- The Core: We scrapped the complex IX trains entirely. In their place, we spec'd six Ecologix HPAF-10000 pressure vessels. These massive units house the bone char media, providing the contact time needed for the fluoride lattice substitution.
- Hydraulics: We cut the final set of booster pumps. Our hydraulic calculations confirmed we had enough head pressure to push the flow all the way to the nearby existing treated water tank, which we are told also houses a disinfection system.
The Bottom Line: Despite the major hardware pivot, the economics remained stable. We held the ROM estimate at $1,952,000.
The Calculus of System Architecture
To manage a 554 GPM flow rate while aggressively reducing fluoride (2.4 mg/L) and molybdenum (0.09 mg/L), we engineered a system defined strictly by mass and dwell time. We moved beyond simple volume sizing and calculated the specific stoichiometric requirements to guarantee performance.
1. Pre-treatment: We selected the Ecologix SM-4824-4A sand filtration skid to handle particulate load. This is a quad-tank array (four 48-inch vessels running in parallel). This selection was driven by surface loading rates.
- Total surface area: Area = 4 x (π x 2²) ≈ 50.27 ft²
- Loading rate: 554 GPM / 50.27 ft² ≈ 11.0 GPM/ft²
By distributing the flow across four tanks, we maintain a conservative loading rate (well below the 15 GPM/ft² limit), ensuring we polish the water without channeling or blowing the media out of the bed.

2. Main treatment: Designing for empty bed contact time (EBCT) post-filtration, we split the flow into two parallel trains to manage head loss. Each train consists of three Ecologix HPAF-10000 pressure vessels in series.
- Flow per train: 277 GPM (≈ 37.03 ft3/min)
- Media mass: 12,000 lbs. per vessel x 3 vessels = 36,000 lbs. per train
To determine the EBCT, we calculated the total media volume per train (assuming a bone char bulk density of ≈ 45 lbs./ft³):
Volume total = 36,000 lbs. / 45 lbs./ft³ = 800 ft³
With the volume and flow rate defined, the dwell time becomes clear:
EBCT = 800 ft³ / 37.03 ft³/min ≈ 21.6 minutes
Standard fluoride removal often attempts to function with 10 to 15 minutes of contact. By engineering for >21 minutes, we maximize the lattice substitution reaction (hydroxyapatite → fluorapatite). Furthermore, this extended contact time negates the competitive interference from molybdate anions, ensuring we hit both contaminant targets.
3. Media volume: 72,000 lbs. of bone char; a calculation of the media's stoichiometric limit against the daily load.
- The Daily Load: We are removing 1.90 mg/L of fluoride (2.4 influent - 0.5 effluent) at 554 GPM. Daily load = (3.02 x 10⁶ L/day) x (1.90 mg/L) ≈ 12.65 lbs. of fluoride / day
- The Theoretical Capacity: Based on the molecular weights of fluoride (38 g/mol) and hydroxyapatite (1004 g/mol), the theoretical saturation point is 3.78% by weight.
- Safety Factor & Run Time: In a passive flow-through system, we apply a safety factor, assuming we achieve ~35% of the theoretical max before breakthrough.
Capacity effective = 72,000 lbs. x 0.0378 x 0.35 ≈ 952 lbs. storage
Run Time = 952 lbs. capacity / 12.65 lbs./day ≈ 75 Days
Conclusion: By utilizing 72,000 lbs. of media, we secured a predictable operational cycle of roughly 2.5 to 3 months.
Hydraulic Validation: The Math Behind the Flow
To confirm we could eliminate the final booster pumps without stalling the system, we performed a Total Dynamic Head (TDH) calculation. We needed to prove that the primary headworks pumps could overcome the friction of three massive media vessels in series and still deliver water to the top of the existing treated water tank.
1. The Specifications
- Target Flow Rate: 554 GPM.
- Piping Specification: 6-inch Schedule 80 PVC (Selected to keep velocity at ~6.3 ft/s and minimize friction loss).
- Static Lift: 10 feet (Height of the open-top discharge into the atmospheric tank).
- Primary Pump Specification: We specified the VFD-equipped skid to deliver a constant discharge pressure of 60 PSI (138 ft. of head).
2. The Energy Equation (Head Loss)
We calculated the cumulative pressure drops (Delta P) across the treatment train to determine the required energy.
- Static Head (Gravity):
Lifting water 10 feet vertically requires 4.33 PSI.
- Piping Friction (~56 ft. Run):
At 554 GPM, 6-inch pipe creates negligible friction, but we accounted for check valves, elbows, and manifolds.
Estimated Loss: 3.0 PSI.
- Sand Filtration Skid (Dirty):
We assumed a "worst-case" scenario where the filters are nearing their backwash setpoint.
Estimated Loss: 10.0 PSI.
- Bone Char Vessels (The Heavy Lift):
This is the critical variable. We have three HPAF-10000 vessels in series per train.
- Vessel 1 (Lead): 7.0 PSI
- Vessel 2 (Lag): 7.0 PSI
- Vessel 3 (Polish): 7.0 PSI
- Total Series Loss: 21.0 PSI.
3. The Conclusion
Result:
The system requires 38.33 PSI to push flow from the headworks to the waterfall discharge at the final tank. Our primary pumps are rated for 60 PSI. We have a safety margin of 21.67 PSI (approx. 36%), confirming that the VFDs can easily drive the entire train in a single pass without secondary amplification.
Engineering the Foundation
You cannot place 200,000 lbs. of operating weight on soft dirt and hope for the best and we have not yet put boots on the ground ourselves, so we specified a foundation capable of handling the static load and the dynamic forces of operation as we had no confirmation of a local civil engineering being involved.
1. Load Profile.
- Total System Mass: 200,000 lbs. (operational)
- Footprint: 40' L x 20' W (800 ft²)
- Distributed Load: We engineered for a uniform load of 250 lbs. per ft² (psf).
2. Slab Architecture.
- 9-inch thick reinforced slab using 4,000 psi concrete.
- Reinforcement: #5 rebar (5/8-inch) laid in a grid pattern at 12 to 18 inches on center.
- The Cage: Crucially, this requires two layers of rebar. One near the top and one near the bottom with 1.5 to 2 inches of concrete cover to prevent corrosion.
- Stress Management: Control joints are required every 10 to 15 feet to manage shrinkage and prevent uncontrolled fracturing.
3. Subgrade & Geotech.
- Bed: A 6 to 12-inch layer of compacted crushed stone is required to maximize load distribution and drainage.
- Soil: The underlying soil must be well-compacted with a minimum bearing capacity of 2,000 psf.
- Rule: If soil conditions are unknown, a geotechnical investigation is critical to confirm bearing capacity and prevent catastrophic settlement.
The Commercial Reality: Silence, then Sign-Off
After the technical breakthrough, we hit the inevitable pause. For five months, the project sat in administrative limbo. Typical.
Then, on July 9, 2025, the silence broke. The University contacted us to pull the trigger. The bureaucratic machinery engaged, the official paperwork was stamped in triplicate, and the project moved from "proposed" to "active." We issued the final official bid at $1,987,000.
By September 2025, the regulatory machine was officially in motion, with licensing and permits making their way through the Ministry of Housing.
It is worth noting that despite the time lapse and the complete overhaul of the process design, our final price landed ~6.5% higher than our original ROM estimate. We didn't just provide a solution; we accurately forecasted the economics from day one.
Glossary of Terms
Activated glass filtration media (AGFM) - A crushed glass media used to replace sand in filtration, offering finer filtration (down to 5 microns) and resistance to bio-fouling.
Bone char - A granular media produced by the carbonization of animal bones, composed primarily of hydroxyapatite and used for fluoride removal via lattice substitution.
Competitive interference - A phenomenon in adsorption where different ions (like molybdenum and fluoride) compete for the same binding sites on the media.
Dissolved air flotation (DAF) - A clarification process that removes suspended matter such as solids or oils by attaching air bubbles to the particles, causing them to float to the surface.
Empty bed contact time (EBCT) - The total time the water is in contact with the media bed, calculated by dividing the volume of the empty bed by the flow rate.
Fiber reinforced plastic (FRP) - A composite material made of a polymer matrix reinforced with fibers, commonly used for corrosion-resistant tanks and vessels.
Fluorapatite - A stable, insoluble mineral (Ca10 (PO4)6F2) formed when fluoride replaces the hydroxyl group in the bone char crystal structure.
Granular activated carbon (GAC) - A highly porous form of carbon used for adsorption of organic contaminants, taste, and odor; often used as pre-treatment to protect resins.
Head pressure - The force exerted by a column of water, used to describe the energy available to push flow through pipes and filters against friction.
Hydroxyapatite - A naturally occurring calcium phosphate mineral (Ca10 (PO4 )6 (OH)2) that forms the structural lattice of bone char.
Ion exchange (IX) - A reversible chemical process where dissolved ions are removed from a solution and replaced with other ions of similar electrical charge.
Lattice substitution - A chemical reaction where an ion in a crystal structure is swapped for another ion of similar size and charge (e.g., fluoride replacing hydroxyl).
Length of unused bed (LUB) - The depth of the filter bed required to contain the mass transfer zone (MTZ); essentially the "safety buffer" of media that is not fully saturated at the moment of breakthrough.
Mass transfer zone (MTZ) - The active region or "wave" within a filter bed where the concentration of the contaminant drops from influent levels to effluent levels.
Nanofiltration - A membrane filtration process that removes particles larger than 1 nanometer, often used for softening water and removing organics.
Peristaltic pump - A type of positive displacement pump used for pumping fluids (often chemicals) by compressing a flexible tube, preventing the fluid from touching pump components.
PID control loop - Proportional-Integral-Derivative controller; a mechanism employing continuous feedback to automatically adjust a process variable (like pH) to a desired setpoint.
Polyaluminum chloride (PAC) - A coagulant widely used in water treatment to destabilize suspended particles so they can be clumped together and filtered out.
Pyrolysis - The thermal decomposition of materials at elevated temperatures in an inert (oxygen-starved) environment.
Rough order of magnitude (ROM) - A high-level cost estimate used in early project stages to determine feasibility before detailed engineering is performed.
Stoichiometric - Relating to the precise quantitative relationships between reactants in a chemical reaction; used here to calculate the theoretical capacity of the media.
Variable frequency drive (VFD) - A controller that drives an electric motor by varying the frequency and voltage supplied to it, allowing for precise control of pump speed and flow.
Frequently Asked Questions
- Why didn't you use synthetic aluminas or membrane technologies (RO)?
Because complexity is the enemy of longevity. While activated alumina is cheaper upfront, it has a fatal flaw: it only works effectively at a pH of 5.5 to 6.0. Using it would have required a similar hazardous acid injection loop that killed the resin option. RO was equally unattractive due to high CapEx, high OpEx, and the significant waste of source water. More importantly, it requires an experienced operator to maintain delicate membranes, and we are in a remote location. Bone char allowed us to treat the water at its natural pH with zero chemical intervention. - What happens to the 72,000 lbs. of media when it's spent? Is it hazardous?
No, it's just rock. The reaction converts the media into fluorapatite, a stable and insoluble mineral identical to the one found in geological formations. Unlike ion exchange resins that vomit out a concentrated liquid brine during regeneration, bone char locks the fluoride into its crystal lattice permanently. When the media is finally exhausted after several months, it is typically non-hazardous solid waste. - You base the media lifespan on a 35% utilization of its theoretical capacity. Is
this an arbitrary safety factor, or is it derived from specific mass transfer
mechanics?
It is a calculation of the Length of Unused Bed (LUB), a foundational concept in fixed-bed adsorption design found in authoritative texts such as Crittenden et al., Water Treatment Principles and Design. The governing principle is that adsorption is not instantaneous. It requires time for the fluoride to diffuse into the bone char pore structure (Intraparticle Diffusion). This creates a long MTZ rather than a sharp boundary. We calculate the operational capacity using the LUB equation: Because the fluorapatite reaction relies on solid-state diffusion, the kinetics are slow, resulting in an elongated MTZ. If we ran to 100% saturation, the "wavefront" of fluoride would exit the vessel days before the media was fully spent. Therefore, we derate the theoretical capacity by 65% to account for this Length of Unused Bed. The 35% figure represents the volume of the bed that is fully saturated before the leading edge of the contaminant wave breaches the 0.5 mg/L effluent limit. - Why did you downgrade the pre-treatment from GAC to sand filtration?
Because bone char isn't a diva. Ion exchange resins are incredibly sensitive to oxidation and organic fouling, which is why we originally specified GAC to protect them. Bone char, however, is mechanically robust and doesn't suffer from the same fouling risks. We swapped GAC for a quad-tank sand filtration skid solely to handle particulates and protect the bed from physical clogging. - How do you ensure Molybdenum removal when Fluoride is the primary target?
We engineered the clock. In adsorption chemistry, "competitive interference" is a real problem with ions fighting for the same parking spots. Standard systems often run with 10 to 15 minutes of contact time. We deliberately oversized the vessels to achieve an EBCT of >21 minutes. This extended dwell time negates the competitive interference from molybdate anions, ensuring that the lattice substitution reaction has enough time to capture both the fluoride and the molybdenum targets. - Why are you running these vessels so close to their 300 GPM limit?
We aren't. While the system total is 554 GPM, the dual-train configuration splits the hydraulic load to 277 GPM per bank. A single vessel or run in series is optimal at 60 to 300 GPM. We are safely inside the hydraulic window while maximizing the EBCT to >21 minutes for fluoride removal.
References
- Colombian National Standard Ministry of Social Protection & Ministry of Environment, Housing and Territorial Development. (2007). Resolución 2115: Por medio de la cual se señalan características, instrumentos básicos y frecuencias del sistema de control y vigilancia para la calidad del agua para consumo humano. https://minvivienda.gov.co/normativa/resolucion-2115-2007
- Toxicity Standards National Research Council (NRC). (2006). Fluoride in Drinking Water: A Scientific Review of EPA's Standards. Washington, DC: The National Academies Press. https://nap.nationalacademies.org/catalog/11571/fluoride-in-drinking-water-a-scientific-review-of-epas-standards
- Engineering Design & LUB Theory Crittenden, J. C., Trussell, R. R., Hand, D. W., Howe, K. J., & Tchobanoglous, G. (2012). MWH's Water Treatment: Principles and Design (3rd ed.). Hoboken, NJ: John Wiley & Sons. https://www.wiley.com/en-us/MWH%27s+Water+Treatment%3A+Principles+and+Design%2C+3rd+Edition-p-9780470405390
- Process Equipment & Filtration Ecologix Environmental Systems. DAF Dissolved Air Flotation, HPAF Pressure Vessels, & Activated Glass Filtration Media (AGFM). https://ecologixsystems.com/dissolved-air-flotation-system/ and https://ecologixsystems.com/chemicals-filtration-media/activated-glass-filtration-media/
- Ion Exchange Media Purolite Corporation. (2025). Product Data Sheet: A200 Strong Base Anion Gel Resin. https://www.purolite.com/product-pdf/A200.pdf
- Specialty Fluoride Media Ion Exchange (India) Ltd. Technical Data Sheet: INDION RS-F Fluoride Removal Resin. https://ionresins.com/pdf/pds/RS-%20F%20PDS.pdf
- Bone Char History & Chemistry Maier, F. J. (1970). Fluoridation. American Water Works Association (AWWA) Manual of Water Supply Practices. https://webstore.ansi.org/sdo/awwa
- Carbon Filtration Standards American Water Works Association. (2022). AWWA B604-18: Granular Activated Carbon. https://store.awwa.org/AWWA-B604-18-Granular-Activated-Carbon
Have a project you would like to discuss?
Contact Ecologix Environmental Systems today to learn more about our engineered solutions.
Contact Us


