Summary
Blue Baby Syndrome, or methemoglobinemia, is a condition often linked to nitrate contamination in drinking water. This article explores its definition, mechanisms, historical occurrences, challenges, and engineering solutions. By understanding the biochemistry, pollution pathways, and real-world cases, you might appreciate the role of advanced water treatment in prevention.
Introduction
In rural Iowa during the 1940s some infants were turning blue, struggling to breathe, all because of something invisible in their well water. This was the dawn of recognizing Blue Baby Syndrome, a form of methemoglobinemia caused by nitrate pollution.[1] In mid-2024, we were tasked with creating a remediation solution for this same issue at a poultry processing facility in Georgia. This article delves into what we learned about methemoglobinemia, its causes, detailed pollution pathways, notable cases, and the engineering innovations that mitigate risks. This article reflects information available as of August 1, 2025.
What is Methemoglobinemia?
Methemoglobinemia is a blood disorder where hemoglobin, the protein responsible for oxygen transport in red blood cells, is converted into methemoglobin (MetHb), an oxidized form incapable of binding oxygen effectively.[2] Normal blood contains less than 1 percent MetHb, maintained by enzymes like NADH-dependent methemoglobin reductase.
There are two main types:
- Congenital methemoglobinemia: Rare genetic deficiencies in enzymes or abnormal hemoglobin variants.
- Acquired methemoglobinemia: Triggered by external agents, such as nitrates, nitrites, or certain drugs. This is the focus here, particularly in infants, earning the moniker Blue Baby Syndrome due to cyanosis (bluish skin discoloration).
In infants under six months, immature gut flora and lower enzyme activity make them especially susceptible.[3] Symptoms escalate with MetHb levels: mild headache at 10-20 percent, severe cyanosis and fatigue at 20-50 percent, and potentially fatal coma above 50 percent.
Table 1: Methemoglobin Levels and Associated Symptoms
| MetHb Level (%) | Symptoms | Clinical Notes |
|---|---|---|
| <10 | Usually asymptomatic; slight skin discoloration possible | Baseline for monitoring in at-risk populations |
| 10-20 | Headache, lightheadedness, mild cyanosis | Early intervention recommended; oxygen therapy may suffice |
| 20-50 | Dyspnea, tachycardia, severe cyanosis, fatigue | Requires immediate medical attention; methylene blue treatment common |
| >50 | Seizures, coma, arrhythmia, death | Emergency; high mortality without rapid antidote administration |
| >70 | Often fatal | Rare survival; aggressive supportive care essential |
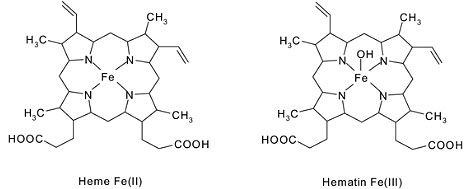
Biochemical Mechanism
At its core, methemoglobinemia from nitrates involves a chain of biochemical reactions. Nitrates (NO3-) in contaminated water are ingested and reduced to nitrites (NO2-) by bacterial nitrate reductase in the gastrointestinal tract, especially in infants with higher gastric pH favoring bacterial growth.
The key reaction is: NO3- + e- → NO2- (catalyzed by nitrate reductase)
Nitrites then enter the bloodstream and oxidize hemoglobin's ferrous iron (Fe2+) to ferric iron (Fe3+), forming MetHb: Hb(Fe2+) + NO2- → MetHb(Fe3+) + NO2 (simplified; actual involves intermediates like nitrosyl hemoglobin)
MetHb cannot release oxygen to tissues, leading to tissue hypoxia despite normal arterial oxygen levels.[3]
Why infants? Their methemoglobin reductase activity is about 50 percent of adults', and fetal hemoglobin is more easily oxidized.[5]
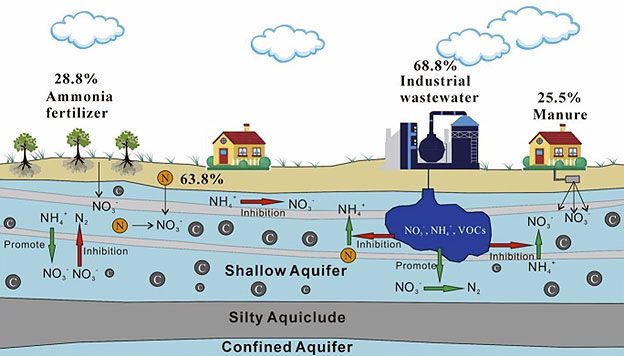
Sources of Nitrate Pollution: How Water Becomes Contaminated
To make the pollution process clear, let's break down the pathways step by step. Nitrate contamination primarily stems from human activity, entering water bodies through diffuse (non-point) and point sources.
- Agricultural Runoff from Fertilizers: Nitrogen-based fertilizers (e.g., ammonium nitrate, urea) are applied to crops. Excess nitrates not absorbed by plants leach into soil. During rainfall or irrigation, they percolate through the vadose zone into groundwater or run off into surface waters. In areas with sandy or permeable soils, leaching is rapid; clay soils slow it but can lead to surface runoff. The EPA estimates that agriculture contributes over 70 percent of nitrate pollution in U.S. waters.[6]
- Manure from Concentrated Animal Feeding Operations (CAFOs): Large-scale livestock operations generate massive manure volumes rich in organic nitrogen. When stored in lagoons or applied to fields as fertilizer, microbial decomposition converts ammonia to nitrates. Over-application or lagoon leaks cause nitrates to infiltrate groundwater. For instance, a single dairy cow produces about 150 pounds of manure daily, containing 0.45 pounds of nitrogen.[7] In regions like the Midwest or Yakima Valley, clustered CAFOs amplify this risk.
- Septic Systems and Wastewater: Faulty septic tanks release untreated sewage, where nitrates form from nitrification of ammonia. Municipal wastewater discharges, if not adequately treated, contribute similarly.
- Other Sources: Industrial discharges (e.g., from explosives or food processing) and atmospheric deposition from fuel combustion add minor loads.
The transport mechanism involves the nitrogen cycle: fixation, mineralization, nitrification (NH4+ → NO2- → NO3- by bacteria like Nitrosomonas and Nitrobacter), and leaching. Factors accelerating pollution include over-fertilization, poor irrigation practices, and lack of buffer zones. Engineers model these using tools like the Nitrogen Loss and Environmental Assessment Package (NLEAP) to predict and mitigate.[8]
Where and When Has Methemoglobinemia Happened?
Methemoglobinemia outbreaks tied to nitrates have historically clustered in agricultural regions with shallow wells.
- United States - 1945 Iowa Case: The first documented cases were reported by Dr. Hunter Comly in Iowa, involving two infants who developed cyanosis after consuming formula mixed with well water containing 140 mg/L nitrates. Both recovered with treatment, but this sparked awareness of nitrate risks.[9]
- United States - 1950s Minnesota Outbreak: A report documented 144 cases over 30 months, with 14 fatalities, primarily in rural areas with nitrate levels exceeding 45 mg/L from farm runoff.[10]
- United States - 2024 Lower Yakima Valley Enforcement: In a recent example highlighting ongoing issues, the Department of Justice, on behalf of the EPA, filed a lawsuit in June 2024 against three large dairies in Washington's Lower Yakima Valley for failing to control nitrate contamination from manure practices, violating a 2013 agreement. These dairies, housing over 30,000 animals, were alleged to endanger neighboring wells. By December 2024, a federal judge ordered immediate well testing, provision of alternative water, and groundwater monitoring to address the pollution. As of mid-2025, the case remains active with appeals to the 9th Circuit Court and a temporary truce until August 11, 2025, for negotiations.[11][12][19]
- Foreign Case - Morocco Study (2000s): In rural Moroccan areas, a cross-sectional study found 22 percent higher methemoglobinemia risk in infants exposed to water >50 mg/L nitrates, linked to contaminated wells near agricultural fields.[13]
These cases underscore the global but preventable nature of the issue, often in areas with intensive farming.
Recent Non-Water-Related Cases
While nitrate-induced cases remain rare in monitored U.S. water supplies, acquired methemoglobinemia from drugs or chemicals persists. For instance, 2025 case reports include dapsone-induced methemoglobinemia in a teenager treated for acne, presenting with classic symptoms and requiring methylene blue intervention.[21] Another example is phenazopyridine-related acute hypoxic respiratory failure in an adult with pre-existing conditions, highlighting the risks of over-the-counter urinary analgesics.[22]
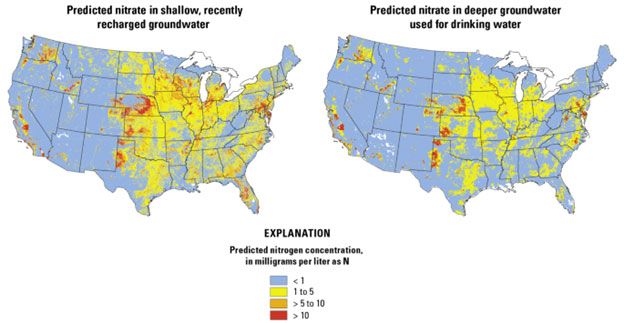
Challenges in Addressing Nitrate-Induced Methemoglobinemia
Detecting nitrate contamination is tricky: it's colorless and odorless, requiring regular testing. Sources like fertilizer runoff are diffuse, complicating regulation. In developing regions, limited infrastructure exacerbates risks. Technically, varying soil types affect nitrate leaching—sandy soils allow faster percolation than clay. Regulatory limits (e.g., EPA's 10 mg/L nitrate-N) are often exceeded in private wells, posing enforcement challenges.[14]
Adding to the complexity, the EPA restarted a reassessment of the health risks of nitrates in drinking water in 2023. While the current standard focuses on preventing methemoglobinemia, emerging evidence links chronic low-level nitrate exposure to increased cancer risks (e.g., colorectal, thyroid), prompting this review. As of March 2025, the EPA determined that the nitrate and nitrite National Primary Drinking Water Regulations (NPDWRs) were not appropriate for revision at this time, but ongoing Integrated Risk Information System (IRIS) assessments may inform future updates. Petitions from organizations, such as Food & Water Watch in May 2025 urging action on agricultural nitrate pollution in groundwater and Earthjustice in July 2025 for immediate abatement in affected communities, highlight calls for stricter standards. Industry groups have downplayed these risks, but the reassessment could eventually lead to stricter standards or additional regulations, impacting engineering designs for treatment systems.[15][16][20][23][24] For global context, the World Health Organization (WHO) sets a guideline of 50 mg/L for nitrate, higher than the EPA's, reflecting varying risk assessments across regions.
Areas Requiring Periodic Updates
Certain aspects of this topic evolve rapidly and warrant regular review:
- Regulatory Standards: EPA and state-level nitrate limits, including ongoing IRIS assessments and responses to petitions.
- Health Links: Emerging research on chronic exposures, such as cancer associations beyond blue baby syndrome.
- Case Studies: New outbreaks or enforcement actions, particularly in agricultural hotspots like the Midwest.
Solutions: Engineering Approaches to Nitrate Removal
Engineering solutions focus on preventing nitrate entry into drinking water through source control and treatment. Common methods include:
- Ion Exchange (IX): Resins selectively swap nitrate ions for chloride or bicarbonate. Process: Water passes through anion-exchange resin beds; regeneration with NaCl brine. Efficiency: >90 percent removal; suitable for municipal systems.
- Reverse Osmosis (RO): Pressure-driven membrane filtration rejects nitrates. Equation for flux: J = A(ΔP - Δπ), where A is permeability, ΔP hydraulic pressure, Δπ osmotic pressure. Ideal for point-of-use but energy-intensive.
- Biological Denitrification: Microbes reduce NO3- to N2 gas in anoxic conditions. Steps: NO3- → NO2- → NO → N2O → N2. Used in advanced wastewater plants.
- Electrodialysis: Electric potential drives ions through membranes; effective but high maintenance.
Companies like Ecologix design and build integrated treatment systems that incorporate these technologies for efficient nitrate removal in wastewater and drinking water applications.[17]
Table 2: Comparison of Nitrate Removal Technologies
| Technology | Removal Efficiency (%) | Cost (Relative) | Pros | Cons |
|---|---|---|---|---|
| Ion Exchange | 90-99 | Medium | High selectivity; easy regeneration | Brine waste disposal needed |
| Reverse Osmosis | 85-95 | High | Removes multiple contaminants | Membrane fouling; energy use |
| Biological Denitrification | 80-95 | Low | Sustainable; low waste | Slower; requires carbon source |
| Distillation | 99+ | High | Complete removal | Energy-intensive; not scalable for large volumes |
| Nanofiltration/Electrochemical Methods | 90-98 | Medium-High | Low energy; modular | Scaling issues |
Emerging Technologies and Future Directions
Nitrate removal technologies in 2025 are evolving toward sustainability. Enhanced biological denitrification uses engineered biofilms to accelerate microbial reduction, improving rates in wastewater systems. Nanotechnology, such as graphene-based adsorbents, offers selective nitrate capture with regeneration potential. For denitrification kinetics, the rate can be modeled as r = μ_max * (NO3- / (K_s + NO3-)) * (C / (K_c + C)), where μ_max is maximum growth rate, K_s is half-saturation constant for nitrate, and C/K_c terms account for carbon source availability.[25] These advancements promise lower energy demands and broader applicability, addressing climate-driven pollution increases.
Conclusion
Methemoglobinemia, nick named Blue Baby Syndrome, highlights the critical link between environmental engineering and health. By grasping its mechanisms, pollution sources, historical and current context, and solutions, engineers can innovate to safeguard water supplies. Our role in designing resilient systems is vital for a safer, bluer planet, one clean drop at a time. You might be wondering whatever happened to that poultry processing facility in Georgia. The solution was adding a denitrification tank to their otherwise robust wastewater treatment plant.
Ready to Optimize Your Water Treatment?
Connect with Ecologix Systems today to discuss your specific needs and discover how our advanced solutions can enhance your operations.
Contact Our Experts NowGlossary

FAQ
- What is the safe nitrate level in drinking water? The EPA sets a maximum of 10 mg/L nitrate-N (equivalent to 45 mg/L NO3-) to prevent methemoglobinemia, though reassessment may address cancer risks.[14]
- How is methemoglobinemia treated medically? Intravenous methylene blue reduces MetHb back to Hb; ascorbic acid (AKA Vitamin C) is used as an adjunct.
- Can adults get Blue Baby Syndrome? Rare, but possible with high exposures.
- What is the best way to solve this issue? Prevention at the source via water treatment.
- What are the specific advantages to using biological denitrification? Biological denitrification offers low operational costs, sustainability by converting nitrates to harmless nitrogen gas (N2), minimal waste production compared to ion exchange, and compatibility with large-scale wastewater treatment systems. It leverages natural microbial processes, reducing chemical use and energy demands.[18]
- How do engineers model nitrate pollution? By using tools like NLEAP to simulate leaching and mitigation strategies.
Bibliography
- Overview of historical Blue Baby Syndrome cases in the Midwest, linking to nitrate contamination.
- Details on methemoglobinemia types, symptoms, and oxygen transport issues.
- Explains causes and symptoms of Blue Baby Syndrome, emphasizing nitrate role in infants.
- Reviews global burden and first U.S. cases by Comly in 1945.
- Discusses nitrate sources, mechanisms, and why infants are vulnerable.
- EPA data on nitrate sources, estimating agriculture’s contribution.
- Details manure from CAFOs as a nitrate source.
- NLEAP model for nitrate leaching assessment.
- Details on 1945 Iowa cases.
- Report on Minnesota methemoglobinemia cases in the 1950s.
- DOJ and EPA complaint against Yakima dairies.
- Judge's order in Yakima case.
- Morocco study on methemoglobinemia.
- EPA nitrate standards
- EPA reassessment for cancer risks
- Restart of nitrate health assessment
- Describes integrated wastewater treatment systems, including nitrogen removal technologies
- Comparison of removal technologies
- Update on Yakima dairies criticism in 2025
- EPA decision on nitrate NPDWRs in 2025
- Dapsone-induced case
- Phenazopyridine-induced case
- Petition on nitrate groundwater crisis
- Petition for EPA action on nitrates
- On denitrification kinetics modeling